Our Platform
Seres’ pioneering science, paired with powerful drug development and manufacturing capabilities, underpins its unique therapeutics platform.
Transforming rigorous scientific insights into a potentially groundbreaking new class of medicines
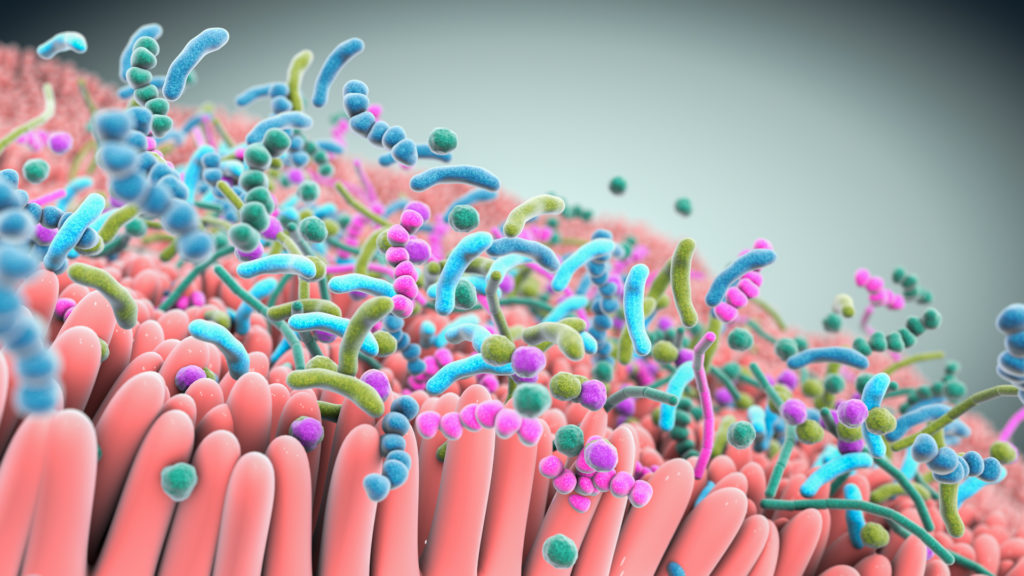
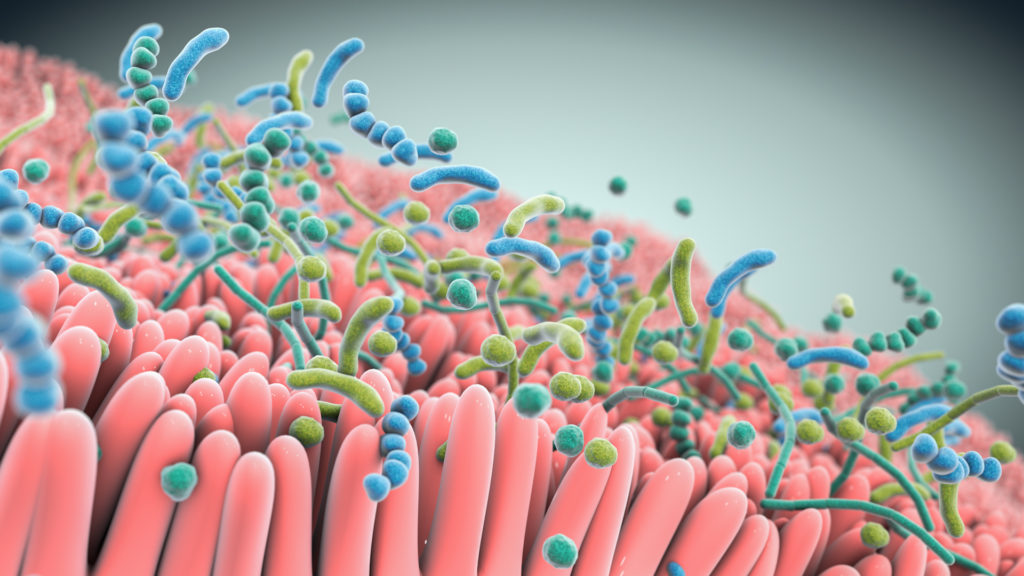
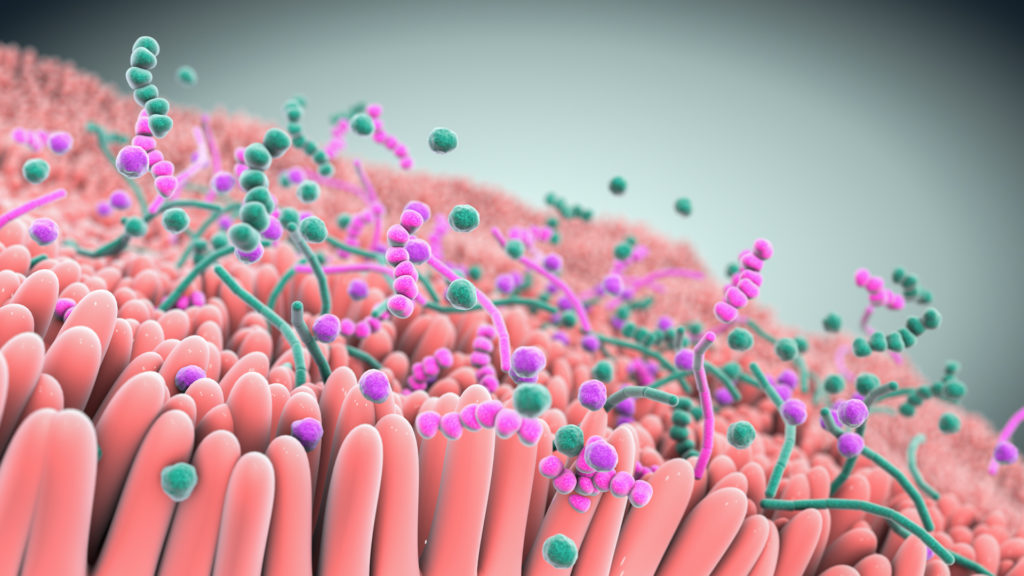
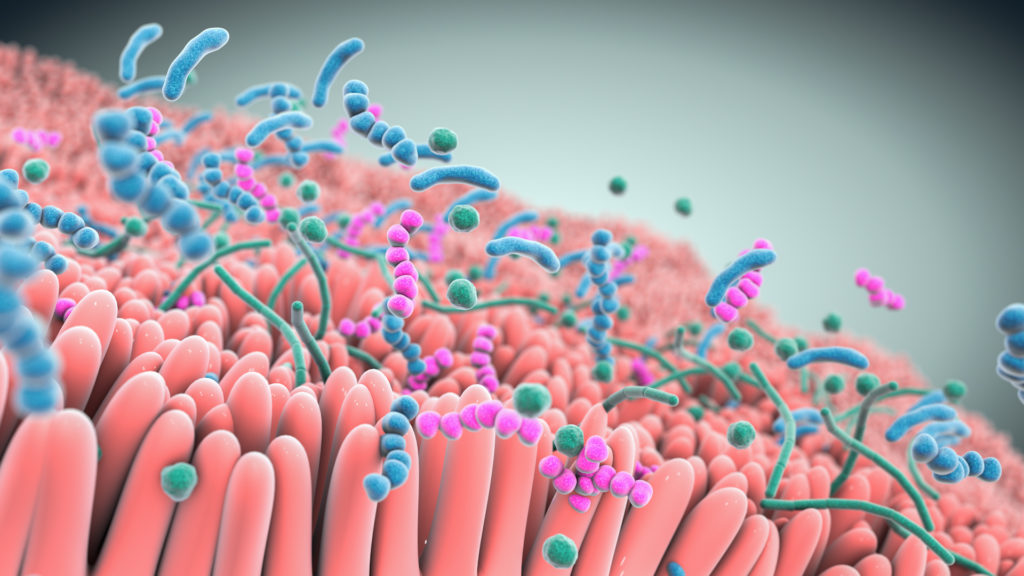
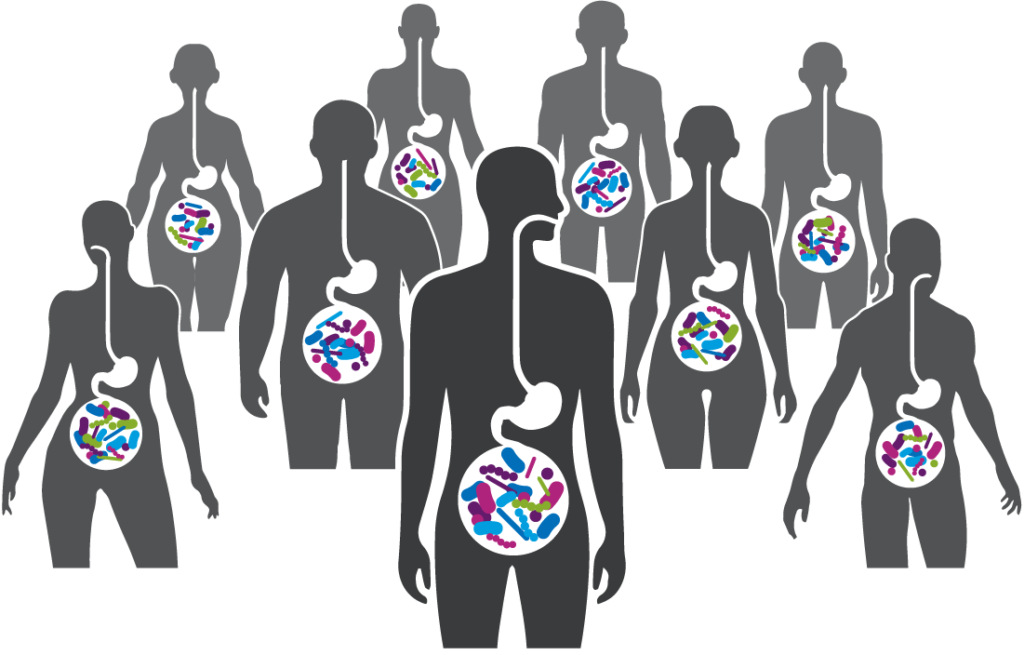
A robust body of research, which includes work by Seres and our collaborators, has revealed the integral role the gut microbiome plays in overall human health. Increasingly the gut microbiome is understood as a pillar of essential functions such as metabolism, immune and inflammatory responses, and protection against potential invaders. Bacteria, a major component of the gut microbiome, can affect these various functions by producing metabolites which interact with other microbes and the host. Scientific understanding of the role of the microbiome and microbe-associated metabolites has advanced tremendously and is allowing development of therapeutics that may treat a variety of serious diseases.
Over the past decade, Seres has pioneered the translation of microbiome insights into an entirely new class of potential new medicines. Our microbiome therapeutics are consortia of bacteria in oral capsules that are designed to have specific functional pharmacological properties to modify the gut microbiome that are being investigated to see if they are able to treat and prevent disease.
Studies show that microbiome therapeutics can drive pharmacological effects across multiple pathways simultaneously.
Our approach:
Delivering a multifunctional
consortium of bacteria
At the heart of our approach are multifunctional consortia of bacteria that are designed to functionally interact with host cells and tissues to treat disease.
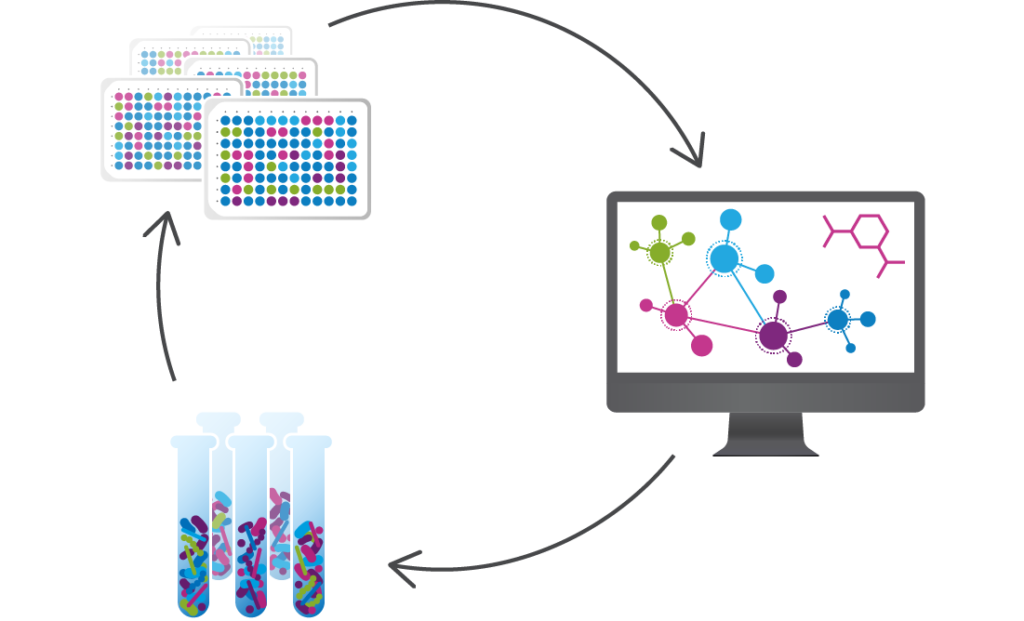
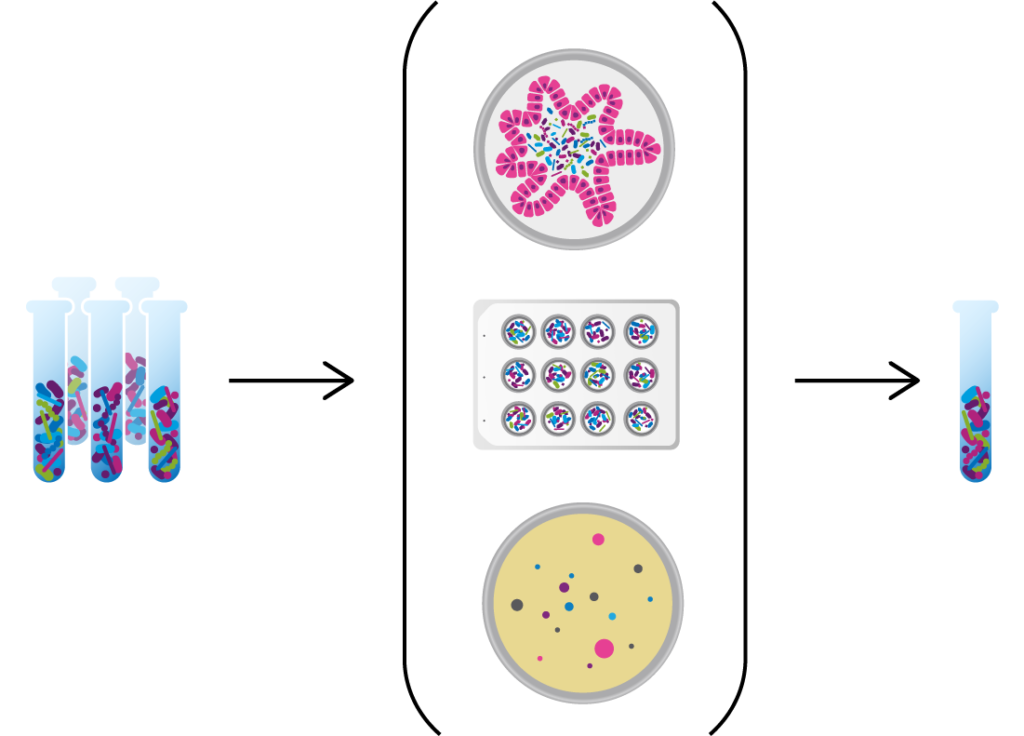
We use data from clinical studies to discover the microbes and microbe-associated metabolites that functionally interact with human cells and tissues to see if they can be targeted to treat diseases, such as ulcerative colitis and C. difficile infection, or to improve the response to disease treatments, such as cancer immunotherapy. We then utilize advanced computational tools and proprietary functional assays to design consortia of commensal bacteria that modulate these targets and act on multiple microbial and host functions.
Our focus on function reflects a key discovery from recent large-scale analyses of the gut microbiome: though microbiome composition can vary widely between healthy individuals, the functional outputs of a healthy microbiome are universal. In other words, good health and resistance to disease depend on the functions provided by the community of microbes, rather than on the presence or absence of any specific bacterial species.
Seres is harnessing defined consortia, or collections, of bacteria to modulate microbiome function. Instead of targeting just one disease pathway, bacterial consortia are multifunctional. This means they might have multiple pharmacological effects and modulate numerous functional pathways within the body to achieve therapeutic impact.
Our strategy:
The Microbiome
Therapeutics Engine
Seres has built an unprecedented Microbiome Therapeutics Engine to design, test, and manufacture multifunctional bacterial consortia.